Development and Validation of UV-Visible Spectrophotometric Method for Simultaneous Estimation of Quercetin and Kaempferol in Bulk Formulation
DOI:
https://doi.org/10.62896/ijpdd.2.7.01Keywords:
Quercetin, Kaempferol, UV-Visible Spectrophotometry, Simultaneous Estimation, Validation, ICH Guidelines.Abstract
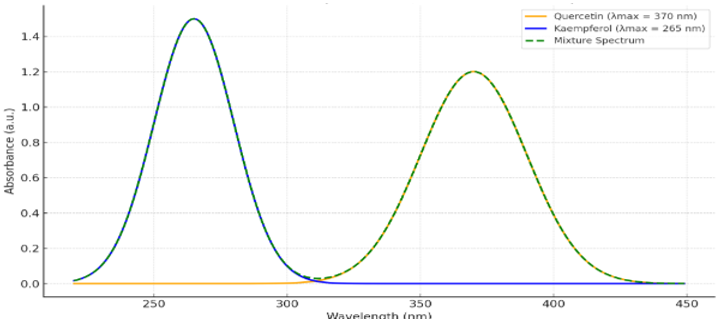
A UV-Visible spectrophotometric method was developed which provides an accurate and economical solution for Quercetin and Kaempferol simultaneous estimation in bulk formulations. The method utilizes an absorbance correction approach to determine the maximum absorption wavelengths of Quercetin at 370 nm and Kaempferol at 265 nm. The solvent used in the experiment was Methanol. The ICH Q2 (R1) guidelines stated that the method should pass validation for linearity along with accuracy, precision, and limit of detection and quantification parameters. The method demonstrated strong linear relationships across the 2–20 μg/mL concentration range for both compounds. The method achieved an accuracy between 98% and 102% while demonstrating acceptable precision with an %RSD of less than 2%. The proposed method enables efficient quality control testing of Quercetin and Kaempferol in combined bulk formulations.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sujata Publications

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.















